Research Overview: Chemical Reactivity at Precision Nanoscale Surfaces
 Innovation in the use of energy resources is a key contemporary challenge because of the significant projected increase in global energy consumption and the pressing demand for disruptive changes in energy utilization to offset the driving forces of climate change. Addressing these needs requires the more efficient use of current petroleum-based energy resources as precursors and fuels, along with increased utilization of alternative energy resources, including biomass and natural gas, and the valorization of readily available but chemically stable potential feedstocks, such as carbon dioxide. Enhancing the viability of alternative energy generation technologies, such as fuel cells, is also required to transform the overall energy landscape. A major prerequisite in meeting each of these scientific challenges is a critical need for the design and synthesis of new highly active and selective catalytic materials.
Innovation in the use of energy resources is a key contemporary challenge because of the significant projected increase in global energy consumption and the pressing demand for disruptive changes in energy utilization to offset the driving forces of climate change. Addressing these needs requires the more efficient use of current petroleum-based energy resources as precursors and fuels, along with increased utilization of alternative energy resources, including biomass and natural gas, and the valorization of readily available but chemically stable potential feedstocks, such as carbon dioxide. Enhancing the viability of alternative energy generation technologies, such as fuel cells, is also required to transform the overall energy landscape. A major prerequisite in meeting each of these scientific challenges is a critical need for the design and synthesis of new highly active and selective catalytic materials.
The study of well-defined materials under complex, working catalytic conditions and the study of complex materials under well-defined, idealized conditions—such as ultrahigh vacuum or computational approaches—both present significant challenges. Enabling the systematic study of catalytic structure-function relationships in these two experimental regimes will drive the development of predictive mechanistic principles that can be applied to the discovery of catalysts for the above transformations. The field of colloidal metal nanoparticle synthesis is just now reaching the point where it is becoming possible to make atomically precise materials with tailored and uniform surface architectures and compositions, though important challenges remain. Our research group has the combination of expertise required to leverage these advances in materials synthesis capabilities to meet this fundamental need in catalyst development.
The Personick Research Group is advancing the state of the art in the chemical synthesis of atomically precise nanomaterials and is using these precision materials to define catalytic structure-function relationships at an elevated level of mechanistic detail. This work is enabled by cutting-edge electron microscopy and spectroscopic techniques, which are also now reaching the level of resolution required for this research.
Area of Impact 1: Electrochemistry as a Design Tool for the Synthesis of Precise Nanomaterials
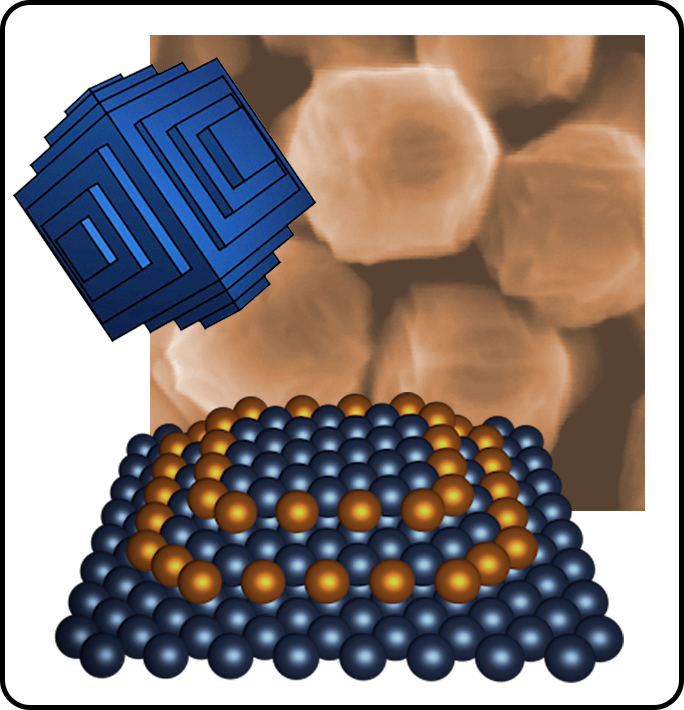
We are developing materials-generalizable chemical tools for controlling the facet structure, composition, and surface ligand environment of metal nanoparticles at the atomic scale. Our group has also pioneered an innovative methodology for using fundamental electrochemical measurements in combination with electrochemically-driven nanoparticle growth in a well-controlled chemical environment to understand redox transformations and the evolution of materials under the complex conditions of colloidal nanoparticle synthesis.
Area of Impact 2: Catalytic Studies on Precision Nanoscale Model Materials
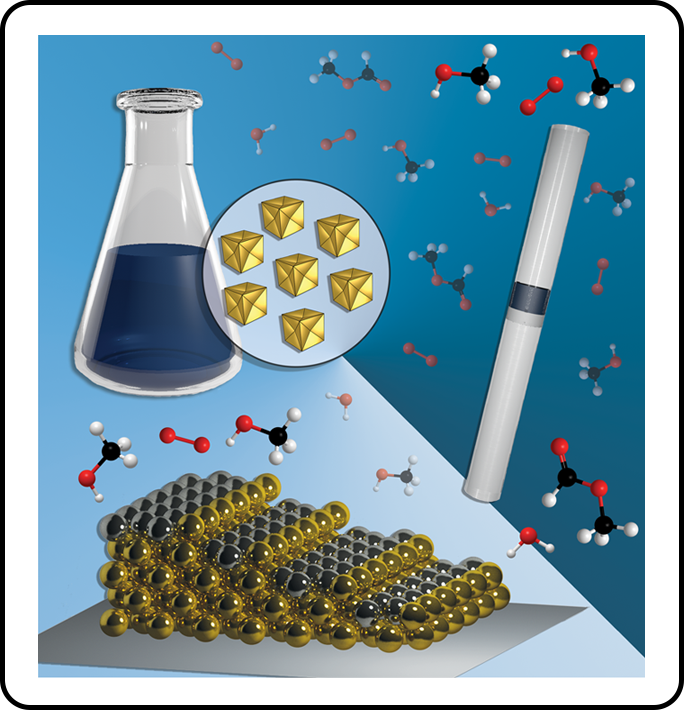
These materials provide a platform for structure-activity studies of catalytic transformations at the intersection of macroscopic single crystal model surfaces and working catalysts, thereby filling the critical “pressure and materials gap” in catalyst design from fundamental principles. Our research group studies these highly precise “nanoscale model surfaces” under realistic catalytic conditions by tracking structure-function relationships resulting from clearly defined modifications of surface atomic arrangement, elemental composition, and the presence of molecular adsorbates.
Area of Impact 3: Plasmon-Mediated Nanoparticle Synthesis and Catalysis
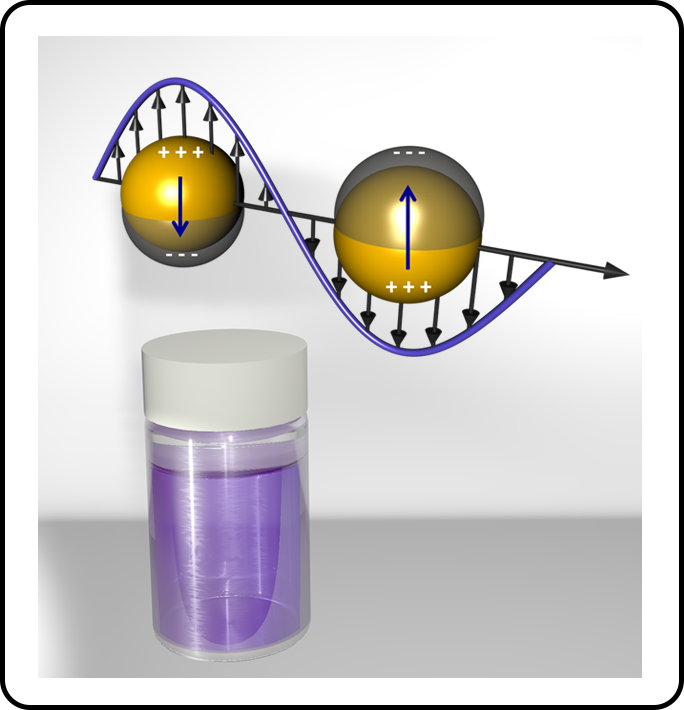
We harness the excitation of non-thermal electron distributions in noble metal nanoparticles and use visible light illumination to achieve reaction selectivity that is not possible via purely thermal reaction chemistry in both materials synthesis and catalytic transformations. We are applying this non-thermal plasmon-driven chemistry to selectively accelerate kinetically slow metal ion reduction processes and overcome key challenges in the synthesis of bimetallic nanoparticles.
