Preprints
- Faulkner, B.; He, Y.; Sitrin, D.; Ziamanesh, L. & Stains, C.I. A Modular Platform for the Optogenetic Control of Small GTPase Activity in Living Cells Reveals Long-Range RhoA Signaling. BioRxiv. DOI: 10.1101/2025.09.07.674731 [Link]
- Zhou, X.^; Belavek, K.J.*; Yin, R.*; Fang, Y.; McAfee, J.L.; Lesiak, L.; Zhang, K.; Cai, M.R.; He, J.; Rabani, E.; Fleming, G.R.; Stains, C.I.^ & Miller, E.W.^ A General Strategy for Enhancing the Brightness of Near-Infrared Fluorophores. ChemRxiv. DOI: 10.26434/chemrxiv-2025-f17vm. ^Co-corresponding authors. *These authors contributed equally to this work. [Link]
Peer-Reviewed Publications
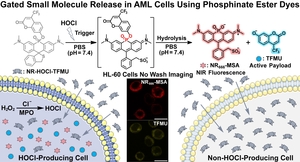
- Fang, Y.*; Zhou, X.*; McAfee, J.L.; Faulkner, B.M.; Lesiak, L.; He, Y.; Brøndsted, F.; Fan, H.; Donarski, E.D.; Hu, X.; Venton, B.J.; Grant, S.; Garrett-Bakelman, F.E. & Stains, C.I. Hypochlorous Acid-Gated Hydrolysis of a Phosphinate Ester Dye in Living Cells. J. Am. Chem. Soc. 147, 40590-40602 (2025). *These authors contributed equally to this work. [Link]
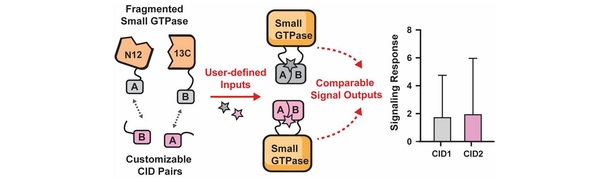
- He, Y.; Faulkner, B.M.; Weatherford, R.S.; Hyun, E. & Stains, C.I. Split-Small GTPase Reassembly as a Method to Control Cellular Signaling with User-Defined Inputs. ACS Chem. Biol. 20, 2049-2055 (2025). [Link]
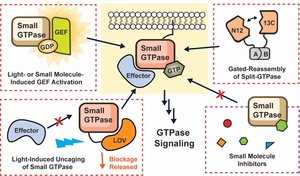
- Faulkner, B.M.; He, Y.; Sitrin, D. & Stains, C.I. Methods for Controlling Small GTPase Activity. ChemBioChem 26, e202500156 (2025). [Link]
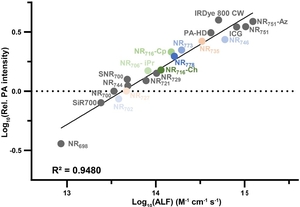
- Brøndsted, F.*; McAfee, J.L.*; Moore, J.D.; Shield, H.R.; Menozzi, L.; Zhou, X.; Fang, Y.; Yin, R.; Yao, J.; Kubelick, K.P. & Stains, C.I. Acoustic loudness factor as an experimental parameter for benchmarking small molecule photoacoustic probes. Nat. Commun. 16, 3779 (2025). *These authors contributed equally to this work. [Link]
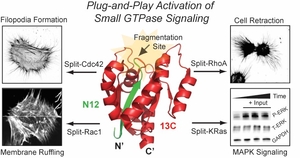
- He, Y.; Faulkner, B.M.; Roberti, M.A.; Bassford, D.K. & Stains, C.I. Standardized Parts for Activation of Small GTPase Signaling in Living Cells. Angew. Chem. Int. Ed. 63, e202403499 (2024). [Link]
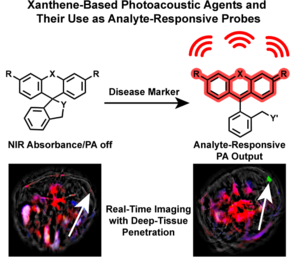
- Brøndsted, F. & Stains, C.I. Xanthene-Based Dyes for Photoacoustic Imaging and Their Use as Analyte-Responsive Probes. Chem. Eur. J. 30, e202400598 (2024). [Link]
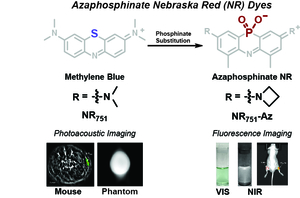
- Yin, R.; Brøndsted, F.; Li, L.; McAfee, J.L.; Fang, Y.; Sykes, J.; He, Y.; Grant, S.; He, J. & Stains, C.I. Azaphosphinate Dyes: A Low Molecular Weight Near-Infrared Scaffold for Development of Photoacoustic or Fluorescence Imaging Probes. Chem. Eur. J. 30, e202303331 (2024). [Link]
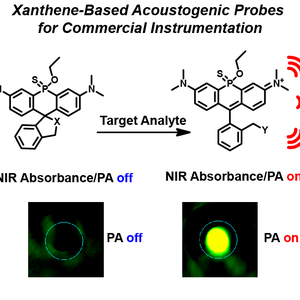
- Brøndsted, F.; Fang, Y.; Li, L.; Zhou, X.; Grant, S. & Stains, C.I. Single Atom Stabilization of Phosphinate Ester-Containing Rhodamines Yields Cell Permeable Probes for Turn-On Photoacoustic Imaging. Chem. Eur. J. 30, e202303038 (2024). [Link]
- Huang, P., Thomas, C.C., Ambati, K., Dholkawala, R., Nagasaka, A., Yerramothu, P., Narendran, S., Pereira, F., Nagasaka, Y., Apicella, I., Cai, X., Makin, R.D., Magagnoli, J., Stains, C.I., Yin, R., Wang, S., Gelfand, B.D. & Ambati, J. Kamuvudine-9 Protects Retinal Structure and Function in a Novel Model of Experimental Rhegmatogenous Retinal Detachment. Invest. Ophthalmol. Vis. Sci. 64, 3 (2023). [Link]
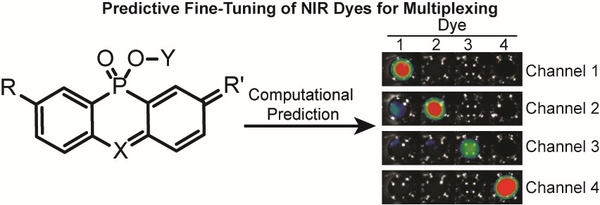
- DiMeglio, D.*; Zhou, X.*; Wirth, T.; Brøndsted, F.; Lesiak, L.; Fang, Y.; Shadmehr, M. & Stains, C.I. Experimentally Calibrated Computational Prediction Enables Accurate Fine-Tuning of Near-Infrared Rhodamines for Multiplexing. Chem. Eur. J. 29, e202202861 (2023). *These authors contributed equally to this work. [Link]
- Zhou, X.; Fang, Y.; Wimalasiri, V.; Stains, C.I. & Miller, E.W. A long-wavelength xanthene dye for photoacoustic imaging. Chem. Commun. 58, 11941-11944 (2022). [Link]
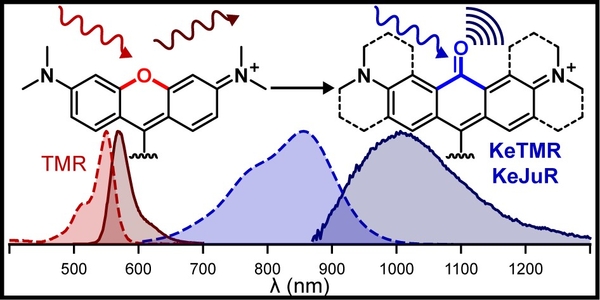
- Brøndsted, F. & Stains, C.I. Heteroatom-Substituted Xanthene Fluorophores Enter the Shortwave-Infrared Region. Photochem. Photobiol. 98, 400-403 (2022). [Link]
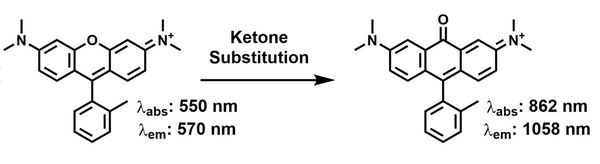
- Ambati, M.; Apicella, I.; Wang, S.-b.; Narendran, S.; Leung, H.; Pereira, F.; Nagasaka, Y.; Huang, P.; Varshney, A.; Baker, K.L.; Marion, K.M.; Shadmehr, M.; Stains, C.I.; Werner, B.C.; Sadda, S.R.; Taylor, E.W.; Sutton, S.S.; Magagnoli, J. & Gelfand, B.D. Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 118 (2021). DOI: 10.1073/pnas.2102975118 [Link]
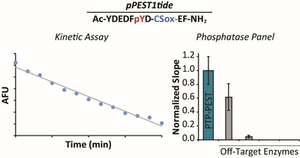
- Casey, G.R. & Stains, C.I. A Fluorescent Probe for Monitoring PTP-PEST Enzymatic Activity. Analyst. 145, 6713-6718 (2020). [Link]
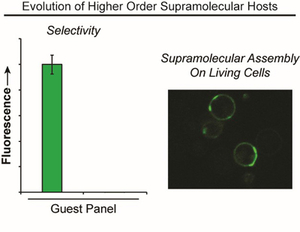
- Casey, G.R.*; Zhou, X.*; Lesiak, L.; Xu, B.; Fang, Y.; Becker, D.F. & Stains, C.I. An Evolutionary Strategy for Identification of Higher Order, Green Fluorescent Host‐Guest Pairs Compatible with Living Systems. Chem. Eur. J. 26, 16721-16726 (2020). *These authors contributed equally to this work. [Link]
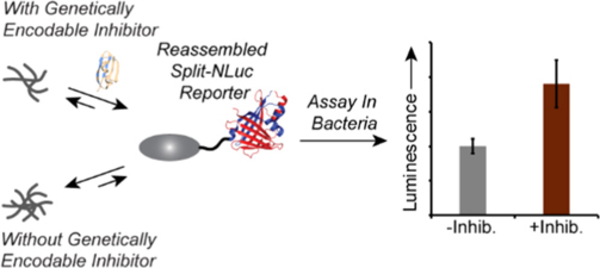
- Nelson, T.J.; Liang, S. & Stains, C.I. A Luminescence-Based System for Identification of Genetically Encodable Inhibitors of Protein Aggregation. ACS Omega 5, 12974-12978 (2020). [Link]
- Yin, R.*; Fang, Y.*; Zhou, X. & Stains, C.I. Synthesis and Application of a Ratiometric Probe for Hydrogen Peroxide. Methods Enzymol. 639, 23-36 (2020). *These authors contributed equally to this work. [Link]
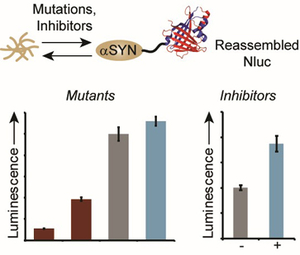
- Nelson, T.J.*; Truong, T.*; Truong, B.; Bilyeu, C. V.; Zhao, J. & Stains, C.I. A Luminescence-Based Assay for Monitoring Changes in Alpha-Synuclein Aggregation in Living Cells. RSC Adv.10, 16675-16678 (2020). *These authors contributed equally to this work. [Link]
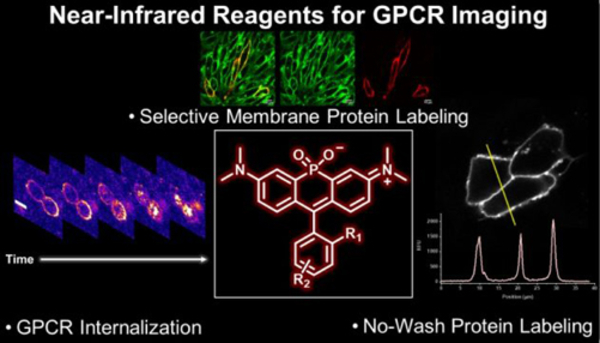
- Lesiak, L.*; Zhou, X.*; Fang, Y.*; Zhao, J.; Beck, J.R. & Stains, C. I. Imaging GPCR Internalization Using Near-Infrared Nebraska Red-Based Reagents. Org. Biomol. Chem. 18, 2459-2467 (2020). *These authors contributed equally to this work. [Link]
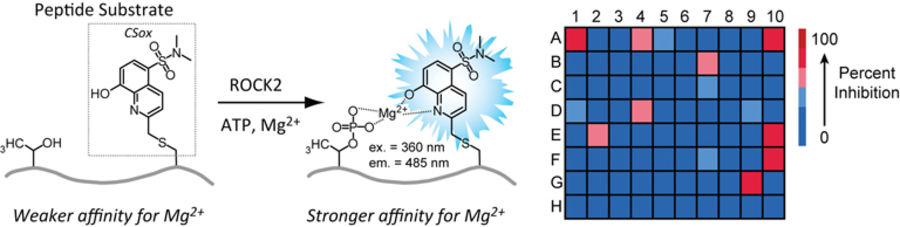
- Beck, J.R.; Cabral, F.; Rasineni, K.; Casey, C.A.; Harris, E.N. & Stains, C. I. A Panel of Protein Kinase Chemosensors Distinguishes Different Types of Fatty Liver Disease. Biochemistry 58, 3911-3917 (2019). [Link]
- Fang, Y.; Good, G.N.; Zhou, X. & Stains, C.I. Phosphinate-Containing Rhodol and Fluorescein Scaffolds for the Development of Bioprobes. Chem. Commun. 55, 5962-5965 (2019). [Link]
- Nelson, T.J.; Zhao, J. & Stains, C.I. Utilizing Split-NanoLuc Luciferase Fragments as Luminescent Probes for Protein Solubility in Living Cells. Methods Enzymol. 622, 55-66 (2019). [Link]
- Casey, G.R.; Beck, J.R. & Stains, C.I. Design and synthesis of fluorescent activity probes for protein phosphatases. Methods Enzymol. 622, 29-53 (2019). [Link]
- Zhou, X; Fang, Y.; Lesiak, L. & Stains, C.I. A Phosphinate‐Containing Fluorophore Capable of Selectively Inducing Apoptosis in Cancer Cells. ChemBioChem 20, 1712-1716 (2019). [Link]
- Casey, G.R. & Stains, C.I. Interrogating Protein Phosphatases with Chemical Activity Probes. Chem. Eur. J. 24, 7810-7824 (2018). [Link]
- Beck, J.R.; Harris, E.N. & Stains, C.I. Quantification of Cell Signaling Networks Using Kinase Activity Chemosensors. Methods Mol. Biol. 1636, 61-70 (2017). [Link]
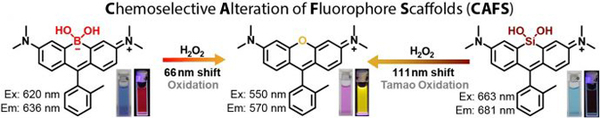
- Zhou, X.; Lesiak, L.; Lai, R.; Beck, J.R.; Zhao, J.; Elowsky, C.G.; Li, H. & Stains, C.I. Chemoselective Alteration of Fluorophore Scaffolds as a Strategy for the Development of Ratiometric Chemodosimeters. Angew. Chem. Int. Ed. 56, 4197-4200 (2017). [Link]
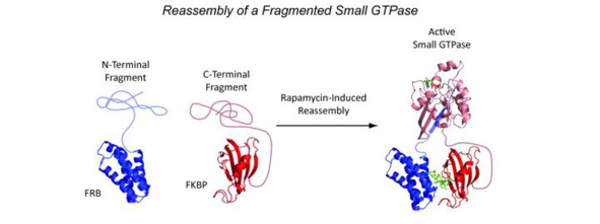
- Zhao, J. & Stains, C.I. Identification of a Fragmented Small GTPase Capable of Conditional Effector Binding. RSC Adv. 7, 12265-12268 (2017). [Link]
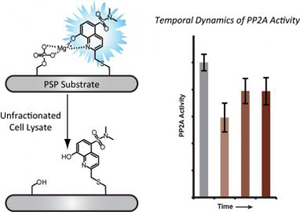
- Beck, J.R.; Truong, T. & Stains, C.I. Temporal Analysis of PP2A Phosphatase Activity During Insulin Stimulation Using a Direct Activity Probe. ACS Chem. Biol. 11, 3284-3288 (2016). [Link]
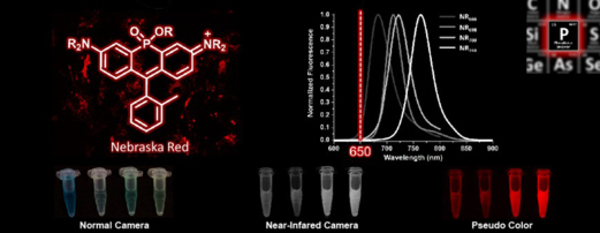
- Zhou, X.; Lai, R.; Beck, J.R.; Li, H. & Stains, C.I. Nebraska Red: A Phosphinate-Based Near-Infrared Fluorophore Scaffold for Chemical Biology Applications. Chem. Commun. 52, 12290-12293 (2016). [Link]
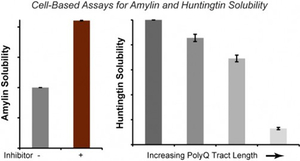
- Zhao, J.; Vu, Q. & Stains, C.I. Luminescent Platforms for Monitoring Changes in the Solubility of Amylin and Huntingtin in Living Cells. Mol. BioSyst. 12, 2984-2987 (2016). [Link]
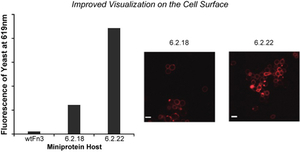
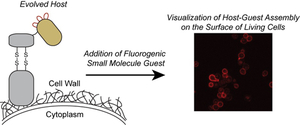
- Xu, B.; Zhou, X. & Stains, C.I. An Improved Miniprotein Host for Fluorogenic Supramolecular Assembly on the Surface of Living Cells. RSC Adv. 6, 20381-20385 (2016). [Link]
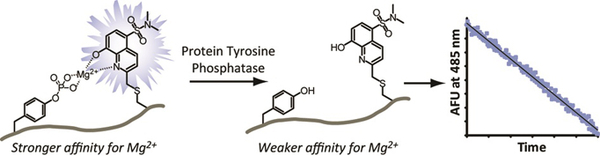
- Beck, J.R.; Lawrence, A.; Tung, A.S.; Harris, E.N. & Stains, C.I. Interrogating Endogenous Protein Phosphatase Activity with Rationally Designed Chemosensors. ACS Chem. Biol. 11, 284-290 (2016). [Link]
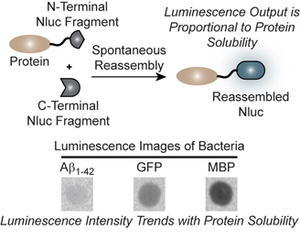
- Zhao, J.; Nelson, T.J.; Vu, Q.; Truong, T. & Stains, C.I. Self-Assembling NanoLuc Luciferase Fragments as Probes for Protein Aggregation in Living Cells. ACS Chem. Biol. 11, 132-138 (2016). [Link]
- Xu, B.; Zhou, X. & Stains, C.I. Supramolecular Assembly of an Evolved Miniprotein Host and Fluorogenic Guest Pair. J. Am. Chem. Soc. 137, 14252-14255 (2015). [Link]
- Beck, J.R.; Zhou, X.; Casey, G.R. & Stains, C.I. Design and Evaluation of a Real-time Activity Probe for Focal Adhesion Kinase. Anal. Chim. Acta 897, 62-68 (2015). [Link]
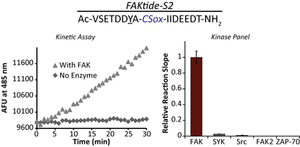
- Kelly, M.I.; Bechtel, T.J.; Reddy, D.R.; Hankore, E.D.; Beck, J.R. & Stains, C.I. A Real-Time, Fluorescence-Based Assay for Rho-Associated Protein Kinase Activity. Anal. Chim. Acta 891, 284-290 (2015). [Link]
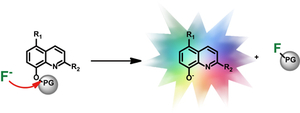
- Zhou, X.; Lai, R.; Li, H. & Stains, C.I. The 8-Silyloxyquinoline Scaffold as a Versatile Platform for the Sensitive Detection of Aqueous Fluoride. Anal. Chem. 87, 4081-4086 (2015). [Link]
- Szalewski, D.A.; Beck, J.R. & Stains, C.I. Design, Synthesis, and Evaluation of a Selective Chemosensor for Leucine-rich Repeat Kinase 2. Bioorg. Med. Chem. Lett. 24, 5648-5651 (2014). [Link]
- Beck, J.R.; Peterson, L.B.; Imperiali, B. & Stains, C.I. Quantification of Protein Kinase Enzymatic Activity in Unfractionated Cell Lysates Using CSox-Based Sensors. Curr. Protoc. Chem. Biol. 6, 135-156 (2014). [Link]
Publications from Postdoc and Grad Student Work
- Stains, C.I.; Tedford, N.C.; Walkup, T.C.; Luković, E.; Goguen, B.N.; Griffith, L.G.; Lauffenburger, D.A. & Imperiali, B. Interrogating Signaling Nodes Involved in Cellular Transformations Using Kinase Activity Probes. Chem. Biol. 19, 210-217 (2012). [Link]
- Stains, C. I.; Luković, E. & Imperiali, B. A p38α-Selective Chemosensor for use in Unfractionated Cell Lysates. ACS Chem. Biol. 6, 101-105 (2011). [Link]
- Furman, J. L.; Mok, P.; Shen, S.; Stains, C. I. & Ghosh, I. A Turn-on Split-Lucif1erase Sensor for the Direct Detection of Poly(ADP-Ribose) as a Marker for DNA Repair and Cell Death. Chem. Commun. 47, 397-399 (2011). [Link]
- Stains, C. I.*; Furman, J. L.*; Porter, J. R.; Rajagopal, S.; Li, Y.; Wyatt, R. T. & Ghosh, I. A General Approach for Receptor and Antibody-Targeted Detection of Native Proteins Utilizing Split-Luciferase Reassembly. ACS Chem. Biol. 5, 943-952 (2010). *These authors contributed equally to this work. [Link]
- Furman, J. L.; Badran, A. H.; Ajulo, O.; Porter, J. R.; Stains, C. I.; Segal, D. J. & Ghosh, I. Toward a General Approach for RNA-Templated Hierarchical Assembly of Split-Proteins. J. Am. Chem. Soc. 132, 11692-11701 (2010). [Link]
- Furman, J. L.; Badran, A. H.; Shen, S.; Stains, C. I.; Hannallah, J.; Segal, D. J. & Ghosh, I. Systematic Evaluation of Split-Fluorescent Proteins for the Direct Detection of Native and Methylated DNA. Bioorg. Med. Chem. Lett. 19, 3748-3751 (2009). [Link]
- Porter, J. R.; Stains, C. I.; Jester, B. & Ghosh, I. A General and Rapid Cell-Free Approach for the Interrogation of Protein-Protein, Protein-DNA, and Protein-RNA Interactions and their Antagonists Utilizing Split-Protein Reporters. J. Am. Chem. Soc. 130, 6488-6497 (2008). [Link]
- Stains, C. I.; Mondal, K. & Ghosh, I. Molecules that Target Beta-Amyloid. ChemMedChem, 2, 1674-1692 (2007). [Link]
- Stains, C. I. & Ghosh, I. When Conjugated Polymers Meet Amyloid Fibrils. ACS Chem. Biol. 2, 525-528 (2007). [Link]
- Porter, J. R.; Stains, C. I.; Segal, D. J. & Ghosh, I. Split b-Lactamase Sensor for the Sequence-Specific Detection of DNA Methylation. Anal. Chem. 79, 6702-6708 (2007). [Link]
- Smith, T. J.; Stains, C. I.; Meyer, S. C. & Ghosh, I. Inhibition of β-Amyloid Fibrillization by Directed Evolution of a β-Sheet Presenting Miniature Protein. J. Am. Chem. Soc. 128, 14456-14457 (2006). [Link]
- Ghosh, I.; Stains, C. I.; Ooi, A. T. & Segal, D. J. Direct Detection of Double-Stranded DNA: Molecular Methods and Applications for DNA Diagnostics. Mol. BioSyst. 2, 551-560 (2006). [Link]
- Stains, C. I.; Furman, J. L.; Segal, D. J. & Ghosh, I. Site Specific Detection of DNA Methylation Utilizing mCpG-SEER. J. Am. Chem. Soc. 128, 9761-9765 (2006). [Link]
- Ooi, A. T.; Stains, C. I.; Ghosh, I. & Segal, D. J. Sequence-Enabled Reassembly of β-Lactamase (SEER-LAC): A Sensitive Method for the Detection of Double-Stranded DNA. Biochemistry 46, 3620-3625 (2006). [Link]
- Ooi, A. T.; Stains, C. I.; Porter, J. R.; Ghosh, I. & Segal, D. J. Sequence-Enabled Reassembly (SEER) Peptides for the Detection of DNA Sequences. Proc. Am. Pep. Soc. 9, 214- 215 (2005). [Link]
- Stains, C. I.; Porter, J. R.; Ooi, A. T.; Segal, D. J. & Ghosh, I. DNA Sequence-Enabled Reassembly of the Green Fluorescent Protein. J. Am. Chem. Soc. 127, 10782-10783 (2005). [Link]