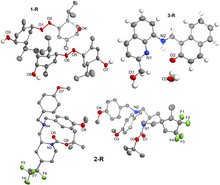
Using the Cambridge Structural Database along with the analysis and visualization programs provided by the Cambridge Crystallographic Data Centre, we identified three organic small molecules with good diffraction data but poorly refined (R1 > 40%) structures. These structures were used as teaching examples in an introductory chemical crystallography course for an activity in which students were asked to identify the errors in the original structure solution or refinement processes. The structures were redetermined, yielding R1 values between 4.04 and 7.04%. In two cases, two chemically equivalent but crystallographically distinct molecules were found in the asymmetric unit, only one of which had been refined in each of the originally published CIFs. In the third case, a false solution found by SHELXS direct methods was corrected and the structure was refined as a hydrated tautomer of what had been originally reported. The identification of this tautomer was supported by theoretical calculations.
Publications
2024
CrI2 is a van der Waals layered material that exhibits helimagnetism that propagates along ribbon chains. This is determined from neutron time-of-flight diffraction measurements. Below TN=17 K in the orthorhombic structure, a screwlike helimagnetic order develops with an incommensurate wave vector of q≈(0.2492,0,0) at 8 K. Using density functional theory (DFT)+U calculations, the J1−J2 model is leveraged to describe the helimagnetism, where J1(>0) and J2(<0) correspond, respectively, to a ferromagnetic nearest-neighbor and antiferromagnetic next-nearest-neighbor intrachain interaction. The DFT+U calculations suggest that orthorhombic CrI2 satisfies conditions that favor formation of helimagnetic order.
The Dirac magnon system CrI3 with a honeycomb lattice is a potential host of topological edge magnons. It ideally orders ferromagnetically (FM) (Tc=61 K) on cooling from a monoclinic (M) to a rhombohedral (R) phase, but antiferromagnetic (AFM) order has been detected in nanometer thin flakes, attributed to M-type layer stacking. There remains confusion, however, as to the extent to which such behavior is present in bulk samples. Using a powder sample in which the sliding transition to the R phase was largely inhibited (2:1 M:R ratio), clear evidence for M-type AFM order (TN∼50 K) coexisting with R-type FM order is observed in the bulk. From inelastic neutron scattering, a lower magnon energy is observed compared to the R phase, consistent with smaller interlayer interactions expected in the M phase. While a gap at the Dirac points has been reported in the R phase, the gap is clearly observed even when the majority is M type, as in our sample, suggesting that the same nontrivial magnon topology of the R phase is present in the M phase as well.
For nearly a century, chemists have explored how transition-metal complexes can affect the physical and chemical properties of linear conjugated polyenes and heteropolyenes. While much has been written about higher hapticity complexes (η4–η6), less is known about the chemistry of their η2 analogues. Herein, we describe a general method for synthesizing 5,6-η2-(1-azatriene) tungsten complexes via a 6π-azaelectrocyclic dihydropyridine ring-opening that is promoted by the π-basic nature of {WTp(NO)(PMe3)}. This study includes detailed spectroscopic and crystallographic data for the η2-dihydropyridine and η2-1-azatriene complexes, both of which were prepared as single regio- and stereoisomers.
Carbon fiber (CF) is a versatile material renowned for its excellent mechanical, thermal, and electrical properties. Polyacrylonitrile (PAN)-based CFs dominate the market due to their high tensile strength, rendering them suitable for structural applications in a wide variety of applications ranging from sporting goods to aerospace. Over five decades of commercial development of PAN-based CF has resulted in a range of varieties with different tensile moduli and tensile strengths. The microstructures, nanostructures, and crystal structures of PAN-based CF play pivotal roles in the macroscale properties of this material. In particular, the crystal structure and crystallite orientation in CF is closely related to the mechanical properties. The crystal structure of PAN-based CF generally consists of turbostratic carbon, which is a disordered form of graphite, the characteristics of which can be effectively characterized in a bulk format through wide-angle x-ray diffraction (WAXD). In this work, we employed a three-part approach to the analysis of WAXD patterns collected from four intermediate modulus PAN-based CFs. The approach incorporates a Scherrer analysis, a Debye analysis, and an orientational analysis to provide precise estimates of crystallite sizes, crystallite distributions, and crystallite orientations with the fiber axis. The results presented here suggest that intermediate modulus PAN-based CF mostly consists of small turbostratic crystallites (<4 nm), with larger crystallites having increased orientation with the fiber axis. The results here imply the presence of curvature and/or wrinkling of the turbostratic layers within the CF structure.
Toxic, hazardous petrochemical solvents are commonly used for industrial-scale ultra-high molecular weight polyethylene (UHMWPE) fiber production, but orange terpenes, a byproduct of orange fruit production, present a bio-derived, sustainable alternative. In this work, fine UHMWPE fibers were spun using orange terpenes as the spin solvent, hot-drawn at a draw ratio of 5:1, investigated for their morphology, microstructure, and thermal and mechanical properties. The resulting fibers exhibited a flat, micro-ribbon cross-section, which is highly desirable for achieving high fiber volume fractions in UHMWPE-fiber reinforced composites. After drawing, the fibers possessed 4× greater breaking tenacity than any previously published studies on UHMWPE fibers spun using orange terpenes with a tenacity of 8.6 cN/dtex and tensile modulus of 229.2 cN/dtex. Microstructural analysis via differential scanning calorimetry and X-ray diffraction revealed that the hot drawing process significantly increased molecular orientation, but crystallinity decreased due to crystallite melting during drawing. Therefore, the mechanical properties of these fibers may be significantly improved with optimization of the fiber drawing process. This work establishes the strong potential of orange terpenes as an environmentally-friendly alternative solvent for UHMWPE gel spinning and sets a foundation for future parametric optimization of the spinning and drawing of these fibers.
Systems that possess open- and closed-shell behavior attract significant attention from researchers due to their inherent redox and charge transport properties. Herein, we report the synthesis of the first diborepin biradicals. They display tunable biradical character based on the steric and electronic profile of the stabilizing ligand and the resulting geometric deviation of the diborepin core from planarity. While there are numerous all-carbon-based biradical systems, boron-based biradical compounds are comparatively rare, particularly ones in which the radical sites are disjointed. Calculations using density functional theory (DFT) and multireference methods demonstrate that the fused diborepin scaffold exhibits high biradical character, up to 95%. Use of a nonsterically demanding diaminocarbene promotes the planarization of the pentacyclic framework, resulting in the synthetic realization of a diborepin containing a dibora-quinoidal core, which possesses a closed-shell ground state and thermally accessible triplet state. The biradicals were structurally authenticated and characterized by both solution and solid-state electron paramagnetic resonance (EPR) spectroscopy. Half-field transitions were observed at low temperatures (about 170 K), confirming the presence of the triplet state. Initial reactivity studies of the biradicals led to the isolation and structural characterization of bis(borepin hydride) and bis(borepin dianion).
Neutral 1-boraphenalene displays the isoelectronic structure of the phenalenyl carbocation and is expected to behave as an attractive organoboron multi-redox system. However, the isolation of new redox states have remained elusive even though the preparation of neutral boron(III)-containing phenalene compounds have been extensively studied. Herein, we have adopted an N-heterocyclic carbene ligand stabilization approach to achieve the first isolation of the stable and ambipolar 1-boraphenalenyl radical 1•. The 1-boraphenalenyl cation 1+ and anion 1– have also been electrochemically observed and chemically isolated, representing new redox forms of boraphenalene for the study of non-Kekulé polynuclear benzenoid molecules. Experimental and theoretical investigations suggest that the interconvertible three-redox-state species undergo reversible electronic structure modifications, which primarily take place on the polycyclic framework of the molecules, exhibiting atypical behavior compared to known donor-stabilized organoboron compounds. Initial reactivity studies, aromaticity evaluations, and photophysical studies show redox-state-dependent trends. While 1+ is luminescent in both the solution and solid states, 1• exhibits boron-centered reactivity and 1– undergoes substitution chemistry on the boraphenalenyl skeleton and serves as a single-electron transfer reductant.
Medicinal chemists use vast combinatorial molecular libraries to develop leads for new pharmaceuticals. The syntheses of these compounds typically rely on coupling molecular fragments through atoms with planar (sp2) geometry. These so-called flat molecules often lack the protein binding site specificity needed to be an effective drug. Here, we demonstrate a coupling strategy in which a cyclohexene is used as a linker to connect two diverse molecular fragments while forming two new tetrahedral (sp3) stereocenters. These connections are made with the aid of a tungsten complex that activates anisole toward an unusual double protonation, followed by sequential nucleophilic additions. As a result, either cis- or trans-disubstituted cyclohexenes can be prepared with a range of chemical diversity unparalleled by other dearomatization methods.
Reaction of isoquinoline (iQuin) with cobalt(II) and nickel(II) chloride and bromide produced a family of neutral coordination complexes: (iQuin)2CoX2 [X=Cl (1), Br (2)], (iQuin)4CoX2 (X=Cl (3), X=Br (4)], (iQuin)4NiX2 [X=Cl, (5), X=Br, (6)], and (iQuin)2NiBr2(CH3CN)2 (7). The crystal structures of 1–5 and 7 are reported. The majority of the compounds crystallize with some of
the iQuin ligands undergoing a ~2-fold rotational disorder, likely caused by the nearly equivalent space occupied by the two positions. The disorder is temperature independent and ~50:50 in all cases but one. Magnetic measurements for all seven compounds indicate that their behavior is dominated by single-ion anisotropy effects with indications of antiferromagnetic exchange also present in 1 and 2.