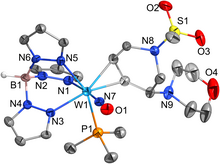
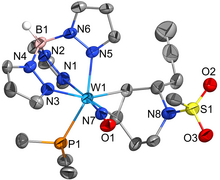
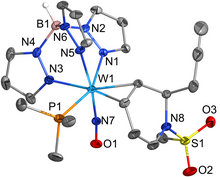
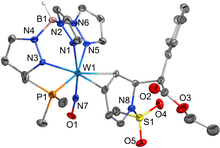
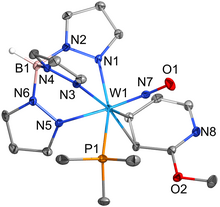
The catalytic reduction of dioxygen (O2) is important in biological energy conversion and alternative energy applications. In comparison to Fe- and Co-based systems, examples of catalytic O2 reduction by homogeneous Mn-based systems is relatively sparse. Motivated by this lack of knowledge, two Mn-based catalysts for the oxygen reduction reaction (ORR) containing a bipyridine-based non-porphyrinic ligand framework have been developed to evaluate how pendent proton donor relays alter activity and selectivity for the ORR, where Mn(p-tbudhbpy)Cl (1) was used as a control complex and Mn(nPrdhbpy)Cl (2) contains a pendent –OMe group in the secondary coordination sphere. Using an ammonium-based proton source, N,N′-diisopropylethylammonium hexafluorophosphate, we analyzed catalytic activity for the ORR: 1 was found to be 64% selective for H2O2 and 2 is quantitative for H2O2, with O2 binding to the reduced Mn(II) center being the rate-determining step. Upon addition of the conjugate base, N,N′-diisopropylethylamine, the observed catalytic selectivity of both 1 and 2 shifted to H2O as the primary product. Interestingly, while the shift in selectivity suggests a change in mechanism for both 1 and 2, the catalytic activity of 2 is substantially enhanced in the presence of base and the rate-determining step becomes the bimetallic cleavage of the O–O bond in a Mn-hydroperoxo species. These data suggest that the introduction of pendent relay moieties can improve selectivity for H2O2 at the expense of diminished reaction rates from strong hydrogen bonding interactions. Further, although catalytic rate enhancements are observed with a change in product selectivity when base is added to buffer proton activity, the pendent relays stabilize dimer intermediates, limiting the maximum rate.
Publications
2024
The non-bonding carbone lone pair in geometrically-constrained antimony and bismuth carbodiphosphorane complexes readily complexed AuCl to afford rare examples of geminal bimetallic carbone coordination featuring a main-group metal.
A family of 6-X-2-hydroxyprydine/pyridone (6-X-2-HOpy/pyridone) Cu(II) compounds, [Cu(6-X-2-HOpy)2Cl2] (1 X = F; 2 X = Cl) and [(6-X-2-pyridone)CuCl(μ-Cl)]2 (3 X = Cl; 4 X = Br), has been prepared. Solution-based infrared spectra displayed a correlation between tautomeric state, primarily driven by halogen identity, and coordination mode with neutral nitrogen coordination mode favored as Br ≪ Cl < F. The tautomeric state of 6-Cl-2-HOpy is influenced by metal ion concentration (M) with lactam concentration increasing as M increases. Compound 1 has F–F contacts less than the sum of the van der Waals radii but falls outside of the typical halogen bonding angle parameters, R–X•••Y = R–Y•••X = 138.2°. Compounds 1 and 2 exhibit weak antiferromagnetic exchanges, fit with a one-dimensional quantum Heisenberg antiferromagnetic linear chain (1D-QHAF) model and J/kB = −1.99(1) K and −0.92(7) K, respectively. Compounds 3 and 4 exhibit a dominating ferromagnetic exchange and an antiferromagnetic exchange and were qualitatively fit to a ferromagnetic linear chain with an interchain interaction model. This model does not accurately represent the physical parameters of the system and was used to show that both exchanges exist and are nontrivial.
Substitution of a C=C bond by an isoelectronic B–N bond is a well-established strategy to alter the electronic structure and stability of acenes. BN-substituted acenes that possess narrow energy gaps have attractive optoelectronic properties. However, they are susceptible to air and/or light. Here we present the design, synthesis and molecular structures of fully π-conjugated cationic BN-doped acenes stabilized by carbodicarbene ligands. They are luminescent in the solution and solid states and show high air and moisture stability. Compared with their neutral BN-substituted counterparts as well as the parent all-carbon acenes, these species display improved quantum yields and small optical gaps. The electronic structures of the azabora-anthracene and azabora-tetracene cations resemble higher-order acenes while possessing high photo-oxidative resistance. Investigations using density functional theory suggest that the stability and photo-physics of these conjugated systems may be ascribed to their cationic nature and the electronic properties of the carbodicarbene.
2023
Polyethylene is a promising low-cost alternative precursor material for carbon fiber production, but it has yet to show mechanical properties near or surpassing polyacrylonitrile-derived carbon fibers. The high molecular weight and order of ultra-high molecular weight polyethylene (UHMWPE) may offer a pathway to realizing this promise by enabling long-range graphitic structure formation and superior mechanical properties. The tension applied during the precursor stabilization process is crucial to maintaining the shape of the fibers during the conversion process, but no published study has yet probed the relationship between sulfonation tension and carbon fiber microstructure and mechanical properties. In this work, a logarithmic sweep of tensile stress was applied to UHMWPE fibers during the stabilization process followed by carbonization. Increasing tension significantly reduced fiber shrinkage, resulted in straighter fibers with less severe kink bands, and greatly improved the mechanical properties of the fibers. Raman spectroscopy and X-ray diffraction revealed that in all cases the carbon fibers were largely amorphous, but increasing tension resulted in increased size and alignment of the turbostratic crystallites with the fiber axis. Large voids were present in the sample fibers, so the Griffith-Irwin relation was employed to predict the potential ultimate tensile strength of the fibers with voids reduced to sizes comparable to commercially produced fibers. This work demonstrates the importance of tension applied during the stabilization of polyethylene fibers for carbon fiber production and establishes a framework for achieving high mechanical properties from these precursors.