Due to its efficacy as a dopamine receptor agonist, methylphenidate (MPH) is of interest as a potential therapeutic for cocaine addiction. While numerous derivatives of MPH have been investigated for their potential medicinal value, functionalization of the piperidine ring has not been explored. The pyridine borane ligand in WTp(NO)(PMe3)(η2-pyBH3) is dearomatized by the metal and can be elaborated to the analogous η2-mesylpyridinium complex. Installing a methyl phenylacetate moiety at the C2′ position via a Reformatsky reaction followed by a tandem protonation/nucleophilic addition sequence results in a library of erythro MPH analogues functionalized at the piperidyl C5′ position. The functional group is added chemoselectively to C5′, cis to the methyl phenylacetate. Repeating this procedure with an enantioenriched source of the tungsten reagent results in enantioenriched MPH derivatives. All identities of the newly reported compounds are supported by comprehensive 2D NMR and HRMS data or crystallographic data.
Publications
2023
Reaction of 2-amino-5-iodopyridine (5IAP) with copper(II) bromide or chloride in alcoholic solution led to four coordination complexes: [(5-IAP)2CuCl2](H2O) (1), (5-IAP)2CuBr2 (2), [(5-IAP)2CuBr2]2 (3) and [(5-IAP)3CuCl2](H2O)n (4). The compounds were characterized by single crystal X-ray diffraction and variable temperature magnetic susceptibility measurements. 1 crystallizes as monomeric units with the 5IAP ligands in the syn-conformation and one lattice water molecule. 2 also exhibits syn-conformation 5IAP ligands, but forms a common bromide bibridged dimer structure. 3 is a conformational polymorph of 2 with anti-oriented 5IAP ligands. Long Cu⋯Br contacts link the molecules into chains. 4 crystallizes with 1.67 lattice water molecules, disordered over three positions. All four compounds exhibit strong halogen bonding. Variable field and temperature magnetic data were obtained on 1-3. 1 is paramagnetic, with no indication of magnetic exchange down to 1.8 K. 2 is well modeled as an antiferromagnetic dimer (J/kB = -22 K) with significant antiferromagnetic interactions between the dimers. 3 is well modeled as a uniform antiferromagnetic chain (J/kB = -6 K) with weak interchain interactions.
Reaction of CuX2·nH2O (X = Cl, n = 2; X = Br, n = 0) with 2-amino-5-methylpyridine (5MAP) in acetonitrile yielded [(5MAP)2CuX2]2 (1, Cl; 2, Br). The compounds are isomorphous and crystallize in the monoclinic space group P21/n as bihalide bridged dimers. Variable temperature magnetic measurements show very weak exchange in the two compounds (-2.1(1) K) which may correlate with previously proposed magnetostructural correlations for bihalide bridged Cu(II) dimers. In attempts to prepare 2 in 2-butanone as solvent, [(3-Br-2-Et-5-Mepyrpy)(CH3CO2)4Cu2] (3) was serendipitously isolated (3-Br-2-Et-5-Mepyrpy = 3-bromo-2-ethyl-5-methyl-7H-pyrrolido[2,3-b]pyridinium). The bicyclic ligand was synthesized in situ by condensation of 5MAP with the solvent; the acetate ions were generated by copper(II) catalyzed Baeyer-Villiger oxidation of the solvent. A mechanism for the synthesis is proposed. The resulting Cu-paddlewheel compound exhibits strong (-400 K) antiferromagnetic interactions.
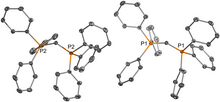
Friedel-Crafts Arylation (the Scholl reaction) is the coupling of two aromatic rings with the aid of a strong Lewis or Brønsted acid. This historically significant C–C bond forming reaction normally leads to aromatic products, often as oligomeric mixtures, dictated by the large stabilization gained upon their rearomatization. The coordination of benzene by a tungsten complex disrupts the natural course of this reaction sequence, allowing for Friedel-Crafts Arylation without rearomatization or oligomerization. Subsequent addition of a nucleophile to the coupled intermediate leads to functionalized cyclohexenes. In this work, we show that by coordinating benzene to tungsten through two carbons (dihapto-coordinate), a rarely observed double protonation of the bound benzene is enabled, allowing its subsequent coupling to a second arene without the need of a precious metal or Lewis acid catalyst.
Three new methoxide-bridged Cu(II) dimers are reported: tetrakis(2-methylpyrazine)di(μ-methoxy)diperchloratodicopper(II) (1), tetrakis(2-amino-5-iodopyridine)dibromide-di-μ-methoxydicopper(II) tetramethanol (2) and catena[(2-amino-5-methylpyridine)μ-bromido-μ-methoxycopper(II) (3). The compounds were characterized by single-crystal X-ray diffraction and variable temperature magnetic measurements. All three compounds crystallize in the triclinic space group P-1, and the dimers lie across a crystallographic inversion center. The dimers in 3 are further bridged into an alternating chain motif via semi-coordinated Cu(II)/Br interactions. All three compounds exhibit strong antiferromagnetic interactions with J/kB = −348(8) K for 1, and the exchange in 2 and 3 being too strong to estimate from the available data. The magnetic data for 1-3 are dominated by trace paramagnetic impurities at temperatures below 200 K. Correlation of the structures with the observed magnetic behavior is proposed.
Superstructures with nanoscale building blocks, when coupled with precise control of the constituent units, open opportunities in rationally designing and manufacturing desired functional materials. Yet, synthetic strategies for the large-scale production of superstructures are scarce. We report a scalable and generalized approach to synthesizing superstructures assembled from atomically precise Ce24O28(OH)8 and other rare-earth metal-oxide nanoclusters alongside a detailed description of the self-assembly mechanism. Combining operando small-angle X-ray scattering, ex situ molecular and structural characterizations, and molecular dynamics simulations indicates that a high-temperature ligand-switching mechanism, from oleate to benzoate, governs the formation of the nanocluster assembly. The chemical tuning of surface ligands controls superstructure disassembly and reassembly, and furthermore, enables the synthesis of multicomponent superstructures. This synthetic approach, and the accurate mechanistic understanding, are promising for the preparation of superstructures for use in electronics, plasmonics, magnetics and catalysis.
Due to the rise in atmospheric carbon dioxide (CO2) concentrations, there is a need for the development of new strategies to enhance the selectivity and activity of the electrocatalytic conversion of CO2 to value-added products. The incorporation of redox mediators (RMs) as cocatalysts to enhance the transfer of redox equivalents during catalysis has been gaining more attention in recent years across a variety of small molecule transformations. We have shown that using Cr-centered complexes with sulfone-based RMs leads to an enhancement of CO2 reduction electrocatalysis under protic conditions via an inner-sphere mechanism. In these cocatalytic systems, an oxygen atom of the reduced RM binds to the Cr center to form a key intermediate stabilized by pancake bonding between the reduced aromatic components of the catalyst ligand backbone and the RM. This interaction facilitates the transfer of an electron and accesses a more kinetically favorable reaction pathway. Here, we show that expanding the aromatic character of the ligand backbone of the catalyst as well as the RM can cause a greater enhancement of coelectrocatalytic activity. These results suggest that further activity improvements can be achieved by focusing on the kinetic and thermodynamic parameters which control association between the catalyst and RM.
Three 4-(2’-pyridyl)imidazole (4-pyim) complexes of copper(II) have been synthesized and studied structurally and magnetically. The structures of [CuCl2(4-pyim)] (1), [CuCl(4-pyim)2]2Cl2(H2O)10 (2), and [Cu(CuCl4)(4-pyim)2][Cu(H2O)(4-pyim)2](CuCl4)(H2O)4 (3) are reported. Single-crystal X-ray diffraction measurements show that 1 crystallizes in the monoclinic space group P21/n with a four-coordinate Cu(II) ion forming dimers via semi-coordinate bonds to bridging chloride ions. The structure of 1 shows the copper and chloride ions disordered over two sites. Compound 2 crystallizes in the triclinic space group P – 1 with five-coordinate Cu(II) ions in a highly distorted geometry between square pyramidal and trigonal bipyramidal. It has an extensive hydrogen bonding network created by 10 lattice water molecules, chloride ions, and nitrogen atoms in the ligands. Compound 3 crystallizes in the monoclinic space group Cc with both four- and five-coordinate Cu(II) ions present in the lattice; the five-coordinate Cu(II) ions display highly distorted geometries. All three compounds have hydrogen bonding and π-stacking interactions among the 4-pyim rings. Magnetic susceptibility data were collected on 1. Magnetic susceptibility data of 1 shows that it exhibits modest antiferromagnetic interactions which are best fit using a honeycomb model [(2J = −2.6(2) K), 2J’ = −1.6(2) K, H = -2JΣsi·sj]. Disorder in the crystal structure decreases the rate of growth of the correlation length at low temperatures, lowering the temperature of the expected maximum in χ below the range of the data.
The syntheses, molecular structures, reactivities, and computational assessment of dipotassium diboratapentacene isomers are described (1 and 2). These compounds represent the first examples of aromatized diboraacenes where the boron atoms are spatially separated in different rings of the acene framework. Both 1 and 2 react with carbon dioxide (CO2) via diastereoselective carboxylation of the pentacene backbone that likely proceeds by a frustrated Lewis pair-like mechanism. The placement of the boron atoms and the reactivity studies provide a platform for later stage functionalization of diboraacenes beyond the central ring of the polycyclic aromatic hydrocarbon core.