Research
Overview
For decades, the dearomatization of arenes has been recognized as a chemical transformation of fundamental importance, providing a pathway from a robust and abundant family of hydrocarbon feedstocks to the alicyclic frameworks found in many biologically active products.
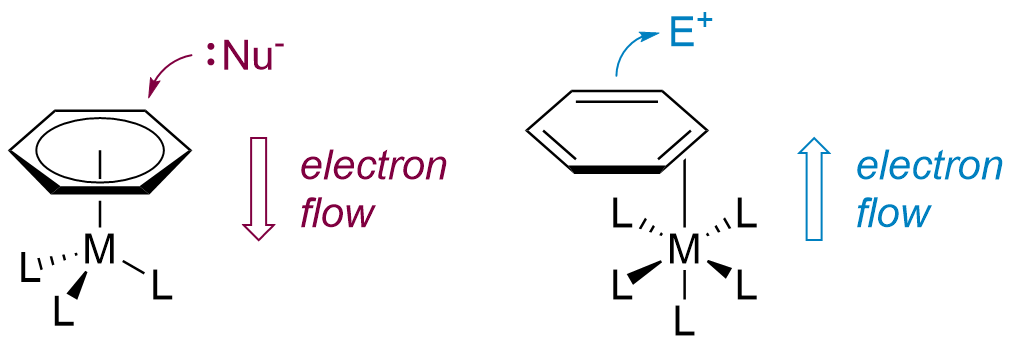
Classically, dearomatization is effected by the binding of an arene to a first-row transition metal, giving complexes such as Cr(CO)3(η6-arene) and Mn(CO)3(η6-arene)+. The bound arenes become susceptible to nucleophilic addition, ultimately yielding substituted arenes or dienes derived thereof, and the metal is typically discarded after this single transformation. The history of this type of dearomatization spans over half a century and is well-established in modern chemistry.
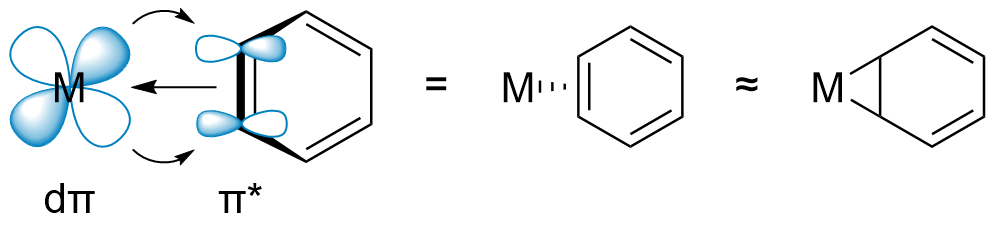
The Harman research group has developed an alternative and complementary approach to dearomatization, in which the arene is bound to the metal (Os, Re, Mo, or W) in a dihapto (η2) manner. Here, the metal-arene bond is primarily stabilized by the interaction of a filled dπ orbital of the metal with an unfilled π* orbital of the arene. This "π back-bonding" activates the bound arene towards electrophilic addition, rather than nucleophilic addition. Furthermore, the increased electron density partially dearomatizes the η2-bound ligand, thus allowing bonds to be formed at the non-coordinated carbons of the π system.
