1) Microdialysis Collection Protocol

Probes & Collection Setup: CMA/10 (Carnegie/Medicin) dialysis probes pictured at left are used in our laboratory to collect dialysates from discrete brain nuclei. The probes have a relative recovery of approximately 20%. The inlet arm of the probe is connected by PE tubing to a 1.0 ul syringe (Exmire/CMA) that is driven by a CMA/100 microinfusion pump. The flow rate of the pump is adjusted to 1.0 ul per minute and the probes are perfused continuously during microdialysis experiments with artificial cerebrospinal fluid (aCSF) (189 mM NaCl, 3.9 mM KCl, 3.37 mM CaCl adjusted to pH 6.3). The outlet arm of the probe is connected by PE tubing to collection vials that contain 0.1 N acetic acid in 13 ul of DHBA (1.5 pg/ul) which serves as an internal standard during HPLC analysis.

Microdialysis Test Chamber: The test chamber consists of a clear plastic bowl (CMA/120 System for Freely Moving Animals) that measures 115 cm in diameter and 35 cm in height. The floor of the chamber is covered with identical shaving chips that are used in the animals home cage. Food and water are available ad libitum during the microdialysis procedures. Collection Procedures: The microdialysis experiments are generally divided into 3 phases consisting of habituation, baseline collection and experimental treatment. Habituation: On the day of testing the animals are transported to the lab and left undisturbed for 20 minutes. Afterwards, the microdialysis probe is inserted through the guide cannula and perfused continuously with aCSF at a flow rate of 1 ul per minute by a CMA/100 microinfusion pump. No microdialysate samples are collected during this one-hour period.Baseline Collection: Baseline collection of dialysate samples generally begin 1 hour and 20 minutes after the start of the experiment. One baseline sample is collected every 20 minutes for a period of 1 hour. Each of these samples contain 20 ul of dialysate and 13 ul of 0.1 N acetic acid and DHBA for a total volume of 33 ul. Experimental Treatment: Drug or placebo injections are initiated two-hours and 20 minutes into the experiment. The substances to be administered and the sequence of their delivery during the treatment period vary in each experiment. Dialysate samples of norepinephrine are collected every 20 minutes. After collection, all samples are sealed with parafilm and stored on ice until they are assayed with HPLC. The entire experiment lasts approximately 4-6 hours.
2) Norepinephrine Assay with HPLC

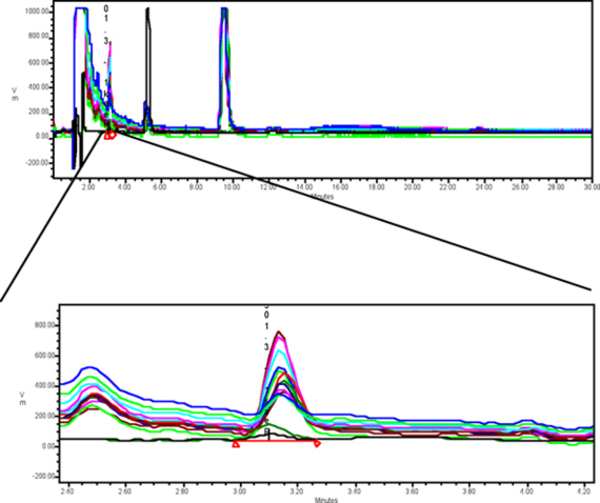
Dialysate samples (33 ul) of norepinephrine are analyzed using reverse-phase liquid chromatography with electrochemical detection (Shown on the left). Norepinephrine is assayed by an HPLC system with a Waters 510 pump, Waters 717 autosampler, Axxiom Chromatography column (5 micron ODS, 25 cm) and an ESA Coulochem Model 5100A electrochemical detector. The mobile phase consist of 50 mg disodium EDTA, 13.8 mg monobasic sodium phosphate and 58 mg octane sulfonate adjusted to pH 3.2 by adding 85% phosphoric acid. The flow rate is adjusted to 0.9 ml per minute. At the end of each experiment the microdialysate samples are loaded into the Waters 717 auto sampler (temperature 7° Celsius) and automatically injected and analyzed with an average analysis period of 35 minutes per sample. The limits of detection for catecholamines with this HPLC system is approximately 2 pg. Norepinephrine concentrations and peak heights are measured in comparison to peak heights and concentration of an internal norepinephrine standard (2, 4, 16 and 20 pg/ul). The concentration, peak height, and retention time for dialysate samples of norepinephrine are saved and stored into a computer by the Waters Millenium database program. A sample chromatogram illustrating the peak heights and retention time of separate norepinephrine standards is shown below.
The findings from a representative in vivo microdialysis study conducted in our lab (Williams, Men, & Clayton, 2002) is shown in the figure below:
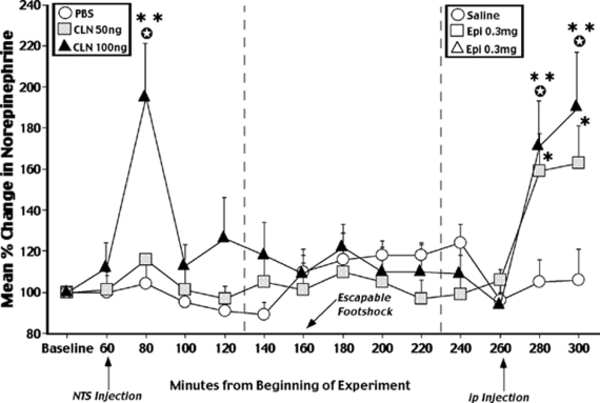
Figure Legend. Mean percent change in amygdala norepinephrine concentrations produced by microinfusion of PBS or clenbuterol (CLN: 50 or 100 ng/0.5 ml) into the NTS, escapable footshock (0.6 mA, 1s), or ip injection of saline or epinephrine (Epi. 0.3 mg/kg). Injection into the NTS of a dose of CLN which improves cognitive functioning (i.e. 100ng) produced a significant increase in amygdala norepinephrine concentrations that exceeded that collected during baseline (** p < .01) or that produced by NTS infusion of PBS (*p < .05). Administration of Epi. in doses that improve memory produced a significant elevation in extracellular norepinephrine concentrations 20 and 40 min post injection (** p < .01, * p < .05). The concentration of norepinephrine sampled following Epi. injection was also significantly greater than that produced by saline (p < .05, t-tests).
