Solid Phase Extraction
New Monolith Stationary Phase for Microfluidic DNA Purification
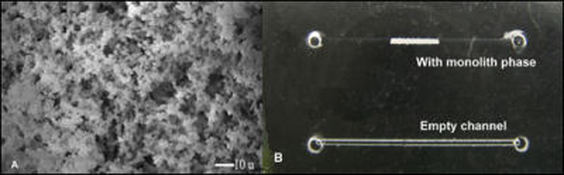
Today, solid-phase extraction (SPE) is the most popular preparation method for the extraction and preconcentration of analytes. To obtain a high loading capacity, a large surface area of the solid phase is desired. Porous polymer monoliths are a new category of materials developed during the last decade. These materials are prepared using a very simple process in which a mixture of monomers and porogenic solvent is polymerized within a closed tuber or other container under carefully controlled conditions. Thus, the monoliths could be prepared into any shape. The polymerization mixture typically contains monomers, free-radical initiator, and porogenic solvent which affords macroporous materials with both large through-pores with a pore size of 1 to 20 nm and small meso-pore in 100-1,000 nm size range. The pore size can also be controlled over a broad range by different ratio of porogenic solvents. Since all the mobile phase must flow through the monolith, the mass transport within the monolith is dominated very much by convection, and the monolithic materials performed very well at high flow rates.
UV-induced photo-polymerization enables the accurate placement of monolithic matrices within the architecture of microscale devices, and the functional surface groups on the monomers allows for easy chemical modification of the surface. These methods have been used to create a high capacity, high efficiency silica-based DNA extraction monolithic column within a microfluidic device.
Microfluidic-based Nucleic Acid Purification in a Two-Stage, Dual-Phase Microchip
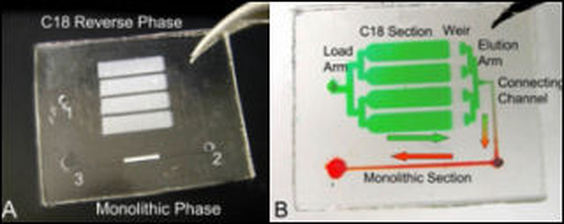
The need for high DNA binding capacity is important in many clinical applications that rely on whole blood as a source of genomic DNA. Lysed whole blood contains nucleic acids, proteins, lipids, metabolites, and inorganic ions, some of which are known to inhibit PCR, a technique used in almost all genetic analyses. However, the proteins in blood also bind to the solid phase limiting the DNA binding capacity. To solve this problem, a two-stage, dual phase microdevice for DNA extraction from whole blood has been developed. This device captures proteins using an in-line C18 phase, allowing the DNA to bind to the DNA extraction phase with significantly decreased protein competition. Successful PCR amplification following purification of DNA from human whole blood illustrated the effectiveness of the method. An added benefit of this method is the ability to remove the protein wash step normally required to remove proteins from the DNA extraction phase; this accelerates the analytical process, reduces the number of steps and eliminates potential sample contamination that may occur from switching syringes or tubing. With the majority of the extracted DNA released in a small volume, this system is ideal for concentrating and purifying DNA from whole blood on integrated microfluidic devices.
Large Volume Reduction Solid Phase Extraction
Often times in forensic cases large volumes are required to elute or solubilize a blood sample from various surfaces including clothing and walls. In contrast, microdevices are characterized by small sample volumes. This raises the need for a sample processing step that would reduce the volume of the forensic sample, providing both a crude purification and sample concentration effect. These investigations focus on a volume reduction microdevice utilizing various solid phases including chitosan-coated-silica particles (charge switch binding) and magnetic silica particles (hydrogen bonding). A comparison of the extraction efficiency of the two phases will be investigated, in addition to optimizing the integration with a second, silica based SPE step. Later work will also involve integration with other processing steps including PCR, ME, and fluorescence detection.


Plastic SPE Microdevices
Silica or sol-gel based solid phases in glass microdevices are normally employed for DNA extractions but have several disadvantages including the difficulty in reproducibly packing the channel and the cost of the glass devices. Polymeric devices provide for a cost effective method to produce microdevices which could be disposable, and current fabrication methods allow for establishment of a polymeric matrix within a microchannel during the fabrication process. This project will investigate the designs utilized in generating a highly reproducible solid phase, and the derivatization of the phase for extraction of DNA from these polymeric devices. This device will be used to perform extractions of samples including blood, cells, frozen tissue, and laser-microdissected histological tissue which can play a major role in cancer research.
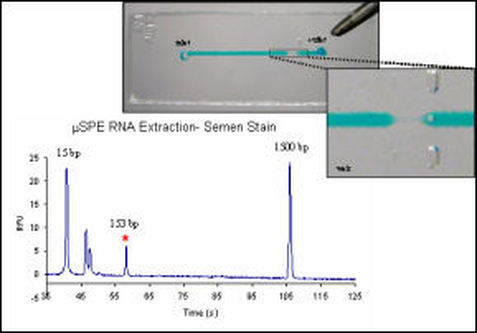
RNA Extraction
Messenger RNA expression analysis requires isolation of RNA from biological samples, followed by reverse transcription-PCR (RT-PCR) amplification and separation of target amplicon to identify the sample. The method is based on the inherently variable mRNA expression from different cell types, producing gene-specific patterns which can be verified by the presence of a unique mRNA expression patterns.Recently, Juusola et al. 1 described a method using mRNA expression to identify specific body fluids. To obtain mRNA for the transcription and amplification necessary for gene expression analysis, RNA must first be isolated and purified from the biological source of interest. Consequently, a robust system for purification of RNA will be essential as the development of messenger RNA expression analysis methods unfold. A closed microfluidic, silica-based purification system would represent a significant improvement to current methodologies for RNA extraction, which often involve time- and reagent-consuming organic extractions, by decreasing the opportunity for introduction of contaminants and RNases, as well as reducing the amount of sample, reagents, and time required to perform this delicate isolation. The application of a silica-based microchip solid-phase extraction method for purification of RNA as a precursor to mRNA profiling would be also be a promising step toward simultaneous DNA and RNA purification technology, permitting a more comprehensive genetic analysis from a single source. In addition, development of a single-process RNA purification device represents the first step towards creation of an integrated micro-total analysis system [μ-TAS] capable of total mRNA profiling, Work with silica and alternative phases to silica for extraction of RNA is underway for use in genetic analysis as well as clinical diagnostics.
1. Juusola, J.; Ballantyne, J. For Sci Internat 2003, 135, 85-96.