
Cell Sorting
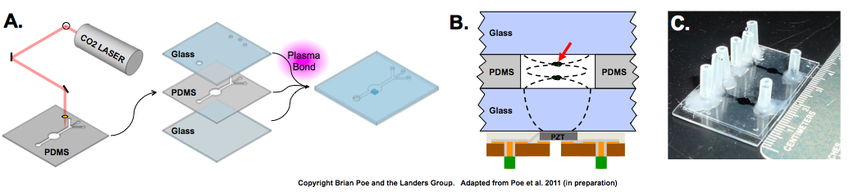
This project highlights the use of acoustic forces in a valveless microfluidic device to trap sperm cells in the presence of female epithelial DNA obtained from sexual assault evidence.The device is comprised of two layers: a printed circuit board layer containing microtransducers, and a glass fluidic layer. An ultrasonic frequency tuned specifically to the transducer characteristics and channel dimensions is applied to the device, and an acoustic standing wave is set up within the microchannel, producing a trapping zone at a pressure node. This method exploits the density, volume, and compressibility differences between sperm cells and free DNA from epithelial cell lysate to create a force strong enough to retain the sperm cells at these nodes, while allowing the free DNA to pass through the device. Laminar flow valving is implemented to direct the two fractions to separate outlets.
Separation and Isolation of Circulating Tumor Cells (CTC) from Whole Blood
Microfluidic acoustic cell separation and sorting (acoustophoresis) is a technique that utilizes ultrasonic standing waves to sort cells based on size and composition as they are passed through a microfluidic channel. A piezoelectric transducer is activated to set up a standing acoustic wave within the microfluidic device creating low pressure nodes through the separation channel. The acoustic forces from the wave focus particles above a desired size into the center of the channel, while smaller particles remain at the edges of the channel. By varying the voltage applied to the transducer, one can change the amplitude of the acoustic forces, resulting in a change in the size of particles focused to the center of the channel.
This technique is currently being applied toward the separation and sorting of circulating tumor cells (CTCs) from whole blood. CTCs vary in size but are generally larger than red blood cells (RBCs), which makes acoustophoresis a great candidate for label free separation and sorting. The force of the acoustic wave moves the larger cells, such as CTCs and white blood cells (WBCs), to the center of the separation channel while the RBCs and other small particles will remain at the outer edges of the channel. This will allow for the isolation and collection of CTCs from RBCs in a label free manner where they can then be collected and studied further.
Enhanced Sperm Cell Recovery from Cotton Swabs for Rape Kit Analysis
Regardless of the method utilized for the separation of vaginal and sperm cell DNA, the overall effectiveness of the procedure is ultimately dependent on the efficiency with which material can be eluted and recovered from a cotton swab. The issue is especially important with swab samples containing low numbers of sperm cells, where any loss makes it even more difficult to obtain a profile of the perpetrator. This project focuses on the development of improved methods for cell elution from a cotton matrix. Several alternative methods of intact cell removal have been investigated; including the use of cellulase-based enzyme mixtures [1], as well as the exclusive use of detergents [2]. These methods can be utilized in conjunction with or to circumvent conventional differential extraction.
1. Voorhees, J.C., Ferrance, J.P., Landers, J.P. Enhanced elution of sperm from cotton swabs via enzymatic digestion for rape kit analysis. J Forensic Sci 2006; 51(3):574-9.
2. Norris, J.V., Manning, K., Linke, S.J., Ferrance, J.P.,Landers, J.P. Expedited, chemically-enhanced sperm cell recovery from cotton swabs for rape kit analysis. J Forensic Sci 2007; 52(4):800-5
